Çocuk Kalp Hastalıkları ve Cerrahisi (Pediatric Heart Diseases and Cardiac Surgery)
Çocuk Kalp Cerrahisi Platformu (Platform for Pediatric Heart Surgery)
Pediatrik Kalp Hastalıkları ve Cerrahisi
Pediatric Heart Diseases and Cardiac Surgery
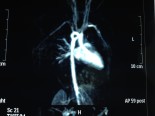
VSD - Ventriküler septal defekt
Ventriküler septal defekt


Doç.Dr.Buğra Harmandar
Ventricular Septal Defect

Pathophysiology
The ventricular septum of the heart is divided into four regions: the inlet (the area beneath the atrioventricular valves), the outlet (the area that gives rise to the great vessels), the perimembranous septum (formed at the junction of the inlet and outlet, adjacent to the tricuspid valve) and the muscular septum (which includes the muscular continuity of the left and right ventricles). Defects in the interventricular septum can occur in any of these four general areas. The most common type of VSD is the perimembranous VSD. Ventricular septal defects result in left to right shunting of blood. The amount of left to right shunting of blood in patients with ventricular septal defect depends upon the size of the defect, the ventricular pressure differential and the relative resistances of the pulmonary and systemic circulations. Isolated non-restrictive VSD results in pulmonary over circulation, left ventricular overload and can lead to left ventricular dilatation and congestive heart failure. In addition, pulmonary vascular obstructive disease can occur.

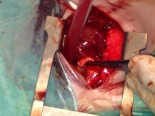
Surgical Technique
Repair of ventricular septal defect requires cardiopulmonary bypass and aortic cross clamping. Most ventricular septal defects are repaired via right atriotomy and are visualized through the tricuspid valve. A patch of synthetic material is utilized to close the defect and is secured with interrupted mattress sutures. Care is taken to avoid atrioventricular conduction tissue as this can result in loss of normal atrioventricular conduction postoperatively. Transesophageal echocardiography is utilized to help assess adequacy of repair. Comparison of mixed venous and pulmonary arterial oxygen saturation is performed to rule out residual ventricular septal defect. Cardiopulmonary bypass time and aortic cross-clamp time required for repair of ventricular septal defects are usually short to moderate in length.

Postoperative Considerations
The postoperative course following isolated VSD closure is usually uncomplicated. Invasive monitors utilized include arterial, central venous and left atrial catheters. A pulmonary artery pressure monitoring catheter is sometimes utilized when preoperative pulmonary artery hypertension exists. Vasoactive infusions required for hemodynamic management might include low dose dopamine or dobutamine, nipride and milrinone. When surgical intervention is delayed in patients with non-restrictive VSD, long-standing pulmonary artery hypertension can result in a more complex postoperative course. Sedation, with infusions of fentanyl and a neuromuscular blocking agent and hyperventilation are instituted to manage pulmonary artery hypertension. Inhaled nitric oxide has proven useful in the reduction of pulmonary vascular resistance and pressure. Atrioventricular conduction abnormalities are occasionally encountered following repair of ventricular septal defects. Edema around the VSD sutures can cause acute development of transient heart block, requiring temporary pacing. Atrioventricular pacing capability should be readily available. Excessive postoperative bleeding is rare. Length of hospital stay required following repair averages five to seven days.