Çocuk Kalp Hastalıkları ve Cerrahisi (Pediatric Heart Diseases and Cardiac Surgery)
Çocuk Kalp Cerrahisi Platformu (Platform for Pediatric Heart Surgery)
Pediatrik Kalp Hastalıkları ve Cerrahisi
Pediatric Heart Diseases and Cardiac Surgery
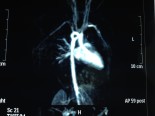
Norwood ameliyatı
Hipoplastik sol kalp sendromunda ilk aşama palyasyon ameliyatıdır.
Doğum sonrası ilk 1-30 günde yapılır.


Doç.Dr.Buğra Harmandar
Norwood Procedure for Hypoplastic Left Heart Syndrome

Pathophysiology
Hypoplastic left heart syndrome occurs when there is failure of adequate development of the systemic or left ventricle. Varying degrees of hypoplasia or atresia of the aorta, mitral valve, and left ventricle may occur. Coarctation of the aorta is a frequent association. The newborn with HLHS is dependent upon a patent ductus arteriosus for systemic perfusion. These patients exhibit unique cardiovascular physiology in which systemic vascular resistance and pulmonary vascular resistance must be carefully manipulated to achieve a balanced circulation with adequate systemic oxygen delivery. Since both the systemic and pulmonary vascular capillary beds are supplied by a single vessel, the pulmonary artery, the relative distribution of blood flow to the systemic and pulmonary circulations is determined by their relative resistances. Without intervention, hypoplastic left heart syndrome is uniformly fatal within the first weeks of life.
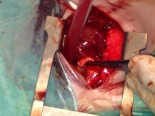
Surgical Technique
Norwood palliation of hypoplastic left heart syndrome requires cardiopulmonary bypass, aortic cross-clamping and hypothermic circulatory arrest. The neo-aorta is constructed utilizing the pulmonary root, ascending aorta, and cryopreserved homograft tissue. The ductal tissue is resected and the augmentation of the aortic arch and site of coarctation is carried out beyond the area of the aortic isthmus. Pulmonary blood flow is provided by placement of a modified Blalock-Taussig shunt. Delayed sternal closure is required in nearly all cases. Cardiopulmonary bypass time and aortic cross-clamp time required to complete Norwood palliation are usually moderate in length. Total circulatory arrest time required for repair is usually less than one hour.
Postoperative Considerations
The postoperative course following Norwood palliation of HLHS can be variable. Invasive monitors utilized postoperatively include arterial, central venous and left atrial catheters. An oximetric catheter is utilized to help assess cardiac output. Vasoactive infusions required for hemodynamic management might include dopamine, epinephrine, milrinone, nitroprusside, phenoxybenzamine and norepinephrine. Infusions of fentanyl and a neuromuscular blocking agent are commonly utilized for sedation following Norwood palliation.
Postoperative bleeding is a common complication following surgery. Numerous suture lines exposed to systemic level pressure predispose these patients to this potential complication. Hemodynamic management following Norwood palliation presents a unique challenge. Knowledge of the interplay between systemic vascular resistance and pulmonary vascular resistance is required to insure a smooth postoperative course. The amount of blood supplied to the pulmonary and systemic circulations is dependent upon their relative vascular resistances. A fall in pulmonary vascular resistance or a rise in systemic vascular resistance will result in pulmonary overcirculation and systemic hypoperfusion. A rise in pulmonary vascular resistance or a fall in systemic vascular resistance results in pulmonary hypoperfusion and cyanosis. Alterations in ventilation, vasoactive infusions, and external stimuli can affect the relative resistances of the systemic and pulmonary circulations. Postoperative management is directed at achieving a balanced circulation with matched pulmonary and systemic flow. Adequacy of systemic oxygen delivery must be carefully monitored.
Arterial oxygen saturation following Norwood palliation is usually seventy to seventy-five percent. Mixed venous oxygen saturation is usually forty-five to fifty-five percent. Lengthy hospital stay following this procedure is common, as these neonates often require careful monitoring of oral and/or nasogastric feedings. Length of hospital stay required following surgery averages two to four weeks.

Yorumlar - Yorum Yaz